A new GLP certification obtained by the Clinical Studies Center (CSC) @ Antibiotice Iași
Our company’s Clinical Research Center (CSC) has received the approval that allows it to continue scientific studies that demonstrate the similarity of generic drugs to original (innovator) drugs, that is, they have the same bioavailability.
CSC studies
Specifically, CSC recently received a new Good Laboratory Practice (GLP) certification from the Romanian National Agency for Medicines and Medical Devices for its bioanalytical laboratory.
This new recertification, valid until 2025, allows the continuation of bioequivalence and phase I clinical studies (studies without therapeutic benefit), which are necessary for the authorization of generic drugs and to ensure the scientific data necessary to confirm their effectiveness and safety in administration to human subjects.
 Our company owns its own Clinical Studies Center, a scientific research unit authorized by ANMDMR since 2006. In the more than 17 years of activity, the center’s specialists have accumulated vast experience embodied in more than 100 studies conducted for external clients from France, Greece, Holland, Norway and Canada.
Our company owns its own Clinical Studies Center, a scientific research unit authorized by ANMDMR since 2006. In the more than 17 years of activity, the center’s specialists have accumulated vast experience embodied in more than 100 studies conducted for external clients from France, Greece, Holland, Norway and Canada.
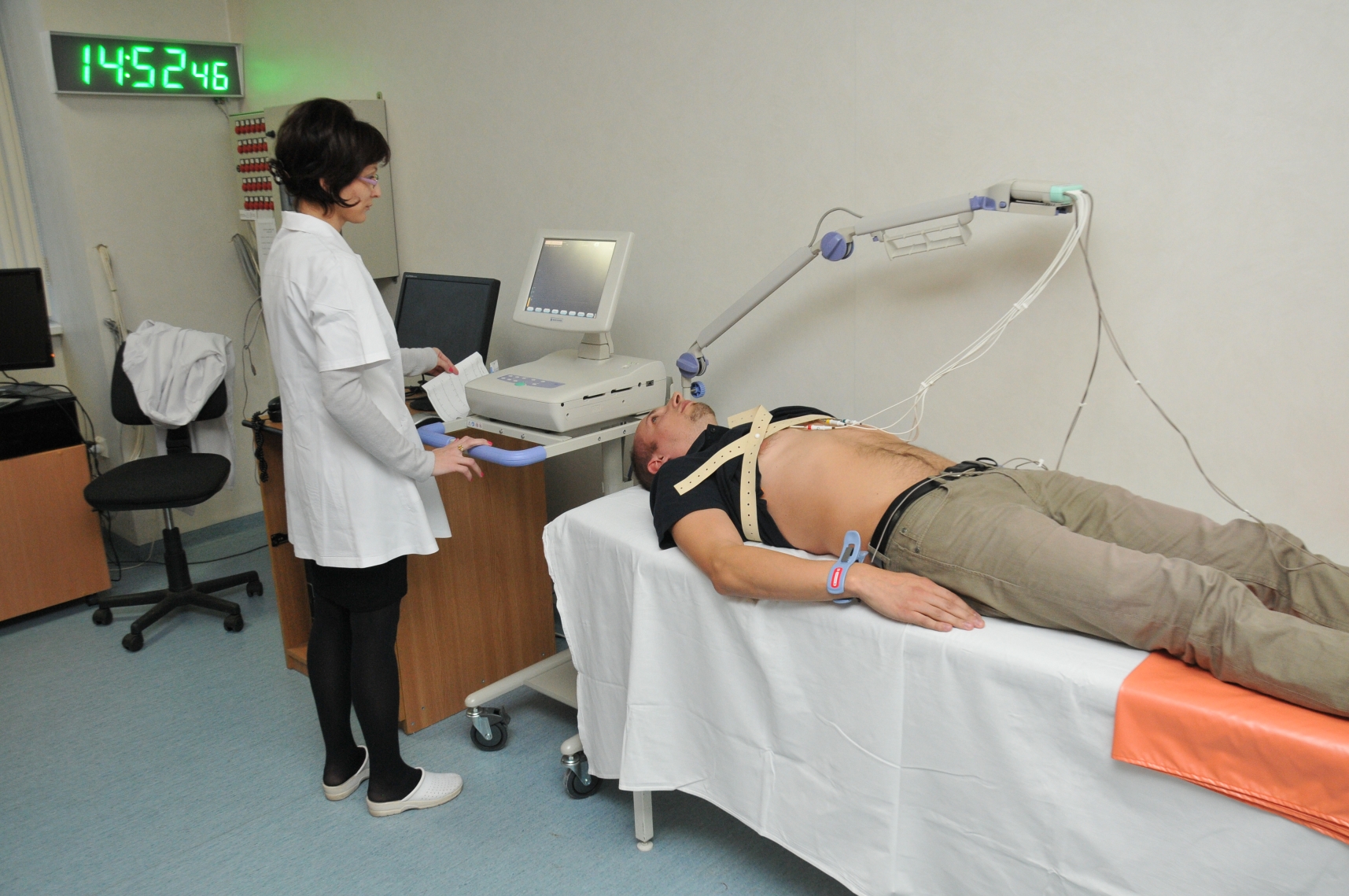 CSC has a clinical unit, a bioanalytical unit and a secondary packaging flow for clinical investigational drugs, all certified according to the applicable standards in the field (GLP, GCP, GMP).
CSC has a clinical unit, a bioanalytical unit and a secondary packaging flow for clinical investigational drugs, all certified according to the applicable standards in the field (GLP, GCP, GMP).
Antibiotice expresses its willingness to offer clinical and/or bioanalytical services specific to phase I clinical trials and bioequivalence studies to interested companies.




