Integrated Management System

At Antibiotice, we place quality, employee safety, and environmental protection at the heart of our operations. That’s why we have implemented an Integrated Management System, which combines quality, environmental, and occupational safety standards across all our processes—from research and production to the distribution of medicines to patients.
Our company’s Integrated Management System is approached by applying the GMP requirements and the three management systems governed by the international standards:
- Quality Management ISO 9001:2015
- Environmental management ISO 14001:2015
- Occupational health and safety management ISO 45001:2018
First certified in 2007 and regularly audited by TÜV Rheinland, this system ensures we uphold the highest pharmaceutical industry standards.
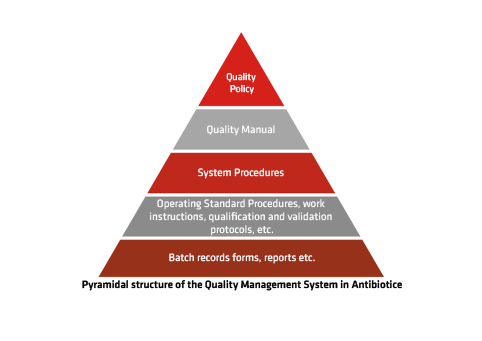
Quality Management – A Continuous Commitment
The Quality Management System, implemented in compliance with EU GMP standards, was the first of its kind in our company and serves as the foundation for all our management systems. It is monitored both internally by our Quality Assurance specialists and externally by business partners and regulatory authorities such as ANMDMR and other international agencies.
Additionally, Antibiotice complies with cGMP USA requirements (CFR 210 & 211), as recognized by FDA inspections in 2013, 2015, and 2017. This certification strengthens our global presence and facilitates collaboration with trusted international partners.
Through our firm commitment to quality and innovation, we ensure that our medicines reach patients safely and effectively.