Contract manufacturing
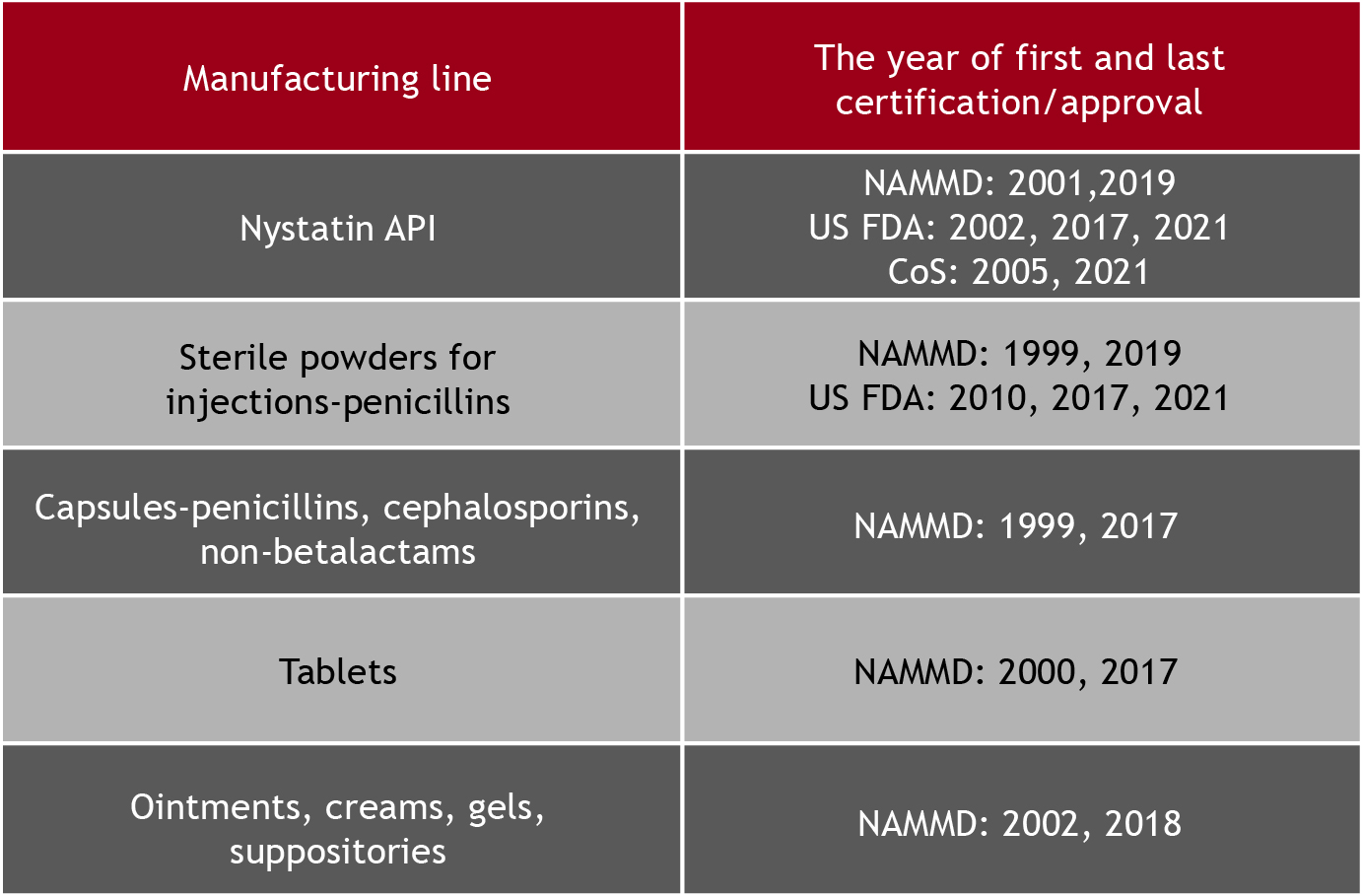
We have certified manufacturing capabilities, our staff is skilled and trained in accordance with GMP requirements, we have expertise and tradition to manufacture in-house high-quality, safe and effective pharmaceuticals for domestic and international partners.
Our company implemented the EU GMP requirements regarding the contract manufacturing and/or control, which allowed us to expanding and diversifying our product portfolio, maintaining the same high standards of quality, safety and efficacy.
The manufacturing agreement and/or control manufacturing agreement must be according to the Marketing Authorization of the product and is concluded between a contract supplier, owner of the Marketing. Authorization and the beneficiary of the contract, the holder of a manufacturing authorization.
The supplier and the beneficiary of the contract both draft and approve, by mutual agreement, a number of specific documents: the confidentiality agreement, the commercial agreement, quality agreement.




